Research Lines
Electrosynthesis of Green H2

H2 Electrosynthesis. Electrosynthesis of green hydrogen is a process that has garnered increasing interest as a sustainable and environmentally friendly means of producing hydrogen gas. This process involves the use of an electrolyzer, which splits water into its constituent elements, hydrogen and oxygen, with the aid of electricity. The source of this electricity is typically renewable energy sources such as solar or wind power, which provides a carbon-neutral method of producing hydrogen. The resulting green hydrogen can be utilized as a clean fuel for various applications, including transportation, energy storage, and industrial processes. Compared to conventional hydrogen production methods that rely on fossil fuels, the electrosynthesis of green hydrogen offers several advantages. These include zero greenhouse gas emissions and the potential for a decentralized production system that can be deployed in remote areas. However, there are still several challenges that need to be addressed in order to scale up the technology and make it economically viable. These challenges include improving the efficiency of the electrolyzer, reducing the cost of production, and integrating the production process with renewable energy sources. Researchers are exploring various approaches to tackle these challenges, including the development of new materials for the electrolyzer, optimizing the operating conditions, and exploring innovative ways to integrate renewable energy sources into the production process. In addition to addressing technical challenges, the electrosynthesis of green hydrogen also faces regulatory and market barriers. Policies and regulations are needed to support the development and deployment of this technology, and there is a need for market incentives to drive the adoption of green hydrogen as a fuel. Despite these challenges, the electrosynthesis of green hydrogen offers a promising pathway towards a more sustainable and low-carbon energy future. With continued research and development, this technology has the potential to become a key contributor to the transition towards a carbon-neutral economy.
Electrosynthesis of Green NH3 and Urea

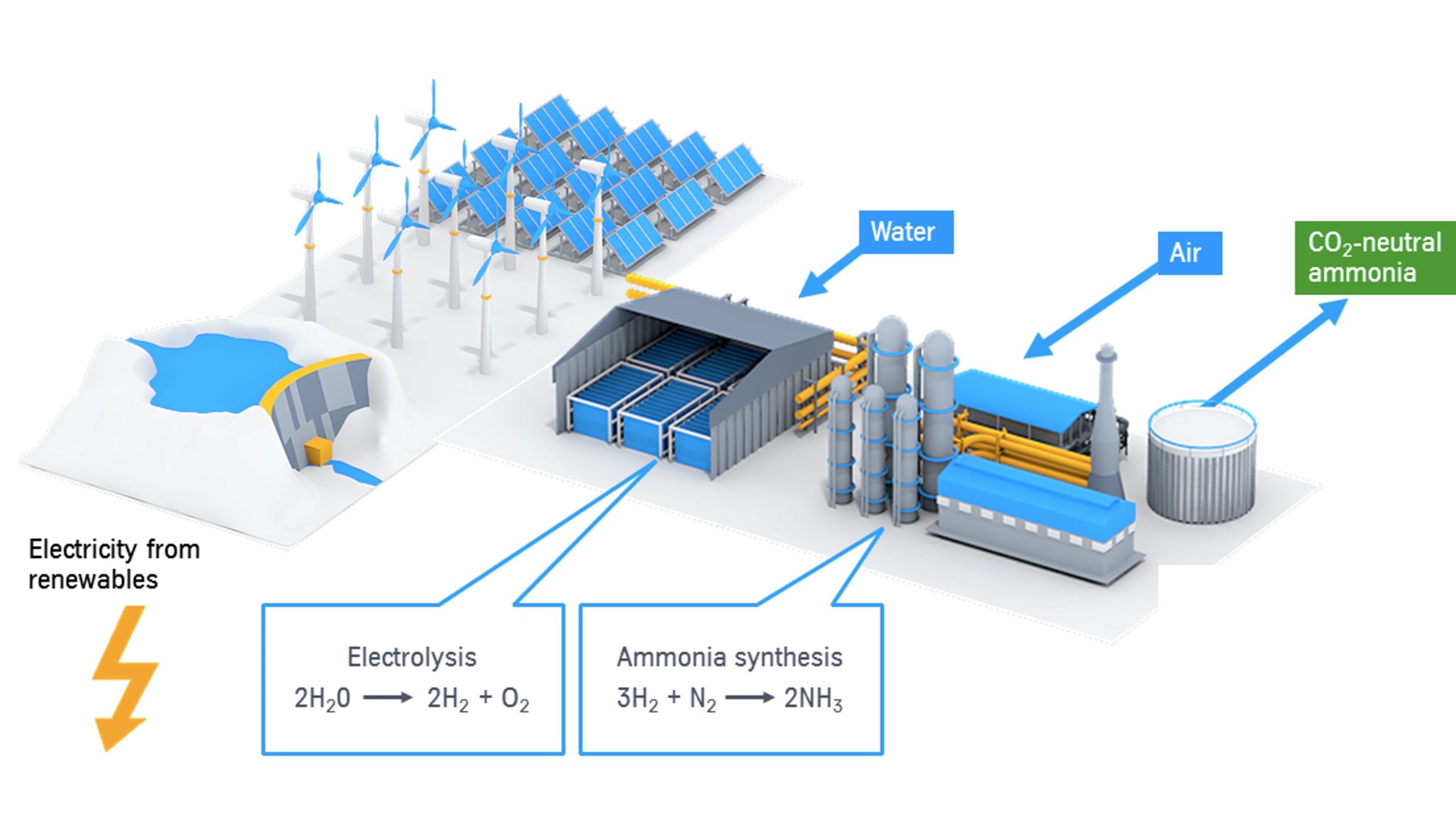
NH3 and Urea Electrosynthesis. Electrosynthesis of ammonia (NH₃) and urea presents a promising pathway toward sustainable chemical production, especially in the context of reducing greenhouse gas emissions and closing the nitrogen cycle. Traditionally, ammonia is produced via the Haber-Bosch process, which operates under harsh conditions and consumes significant amounts of fossil fuels, contributing heavily to global CO₂ emissions. In contrast, electrocatalytic ammonia synthesis enables NH₃ production from nitrogen (N₂) or nitrate (NO₃⁻) under ambient conditions using renewable electricity, offering a cleaner alternative. Similarly, urea — an essential fertilizer and industrial chemical — can be synthesized electrochemically through the co-reduction of carbon dioxide (CO₂) and nitrogenous species such as NO₃⁻ or N₂. This process not only addresses CO₂ utilization but also provides a route to valorize nitrogen pollutants in wastewater. However, both NH₃ and urea electrosynthesis face significant challenges in terms of selectivity, Faradaic efficiency, and reaction kinetics, particularly for the C–N coupling step in urea formation. Advanced electrocatalysts, such as transition metal oxides, single-atom catalysts, and high-entropy alloys, are being developed to overcome these barriers. Gas diffusion electrodes and membrane electrode assemblies (MEA) are increasingly explored to enhance mass transport and enable higher current densities suitable for scaling up. Additionally, in situ and operando techniques are essential to unravel reaction mechanisms and guide rational catalyst design. From an economic perspective, improving energy efficiency and production rates (e.g., >100 mA cm⁻², >10⁻⁶ mol cm⁻² h⁻¹) is crucial for industrial viability. Life cycle assessment (LCA) and techno-economic analysis (TEA) help identify bottlenecks and prioritize research directions. Integrating these technologies with decentralized or modular systems could support on-site fertilizer production, especially in rural and off-grid regions. Ultimately, the electrosynthesis of NH₃ and urea represents a key enabler of green chemistry and circular economy strategies, aligning with global sustainability goals.
Electrochemical Reduction of CO2
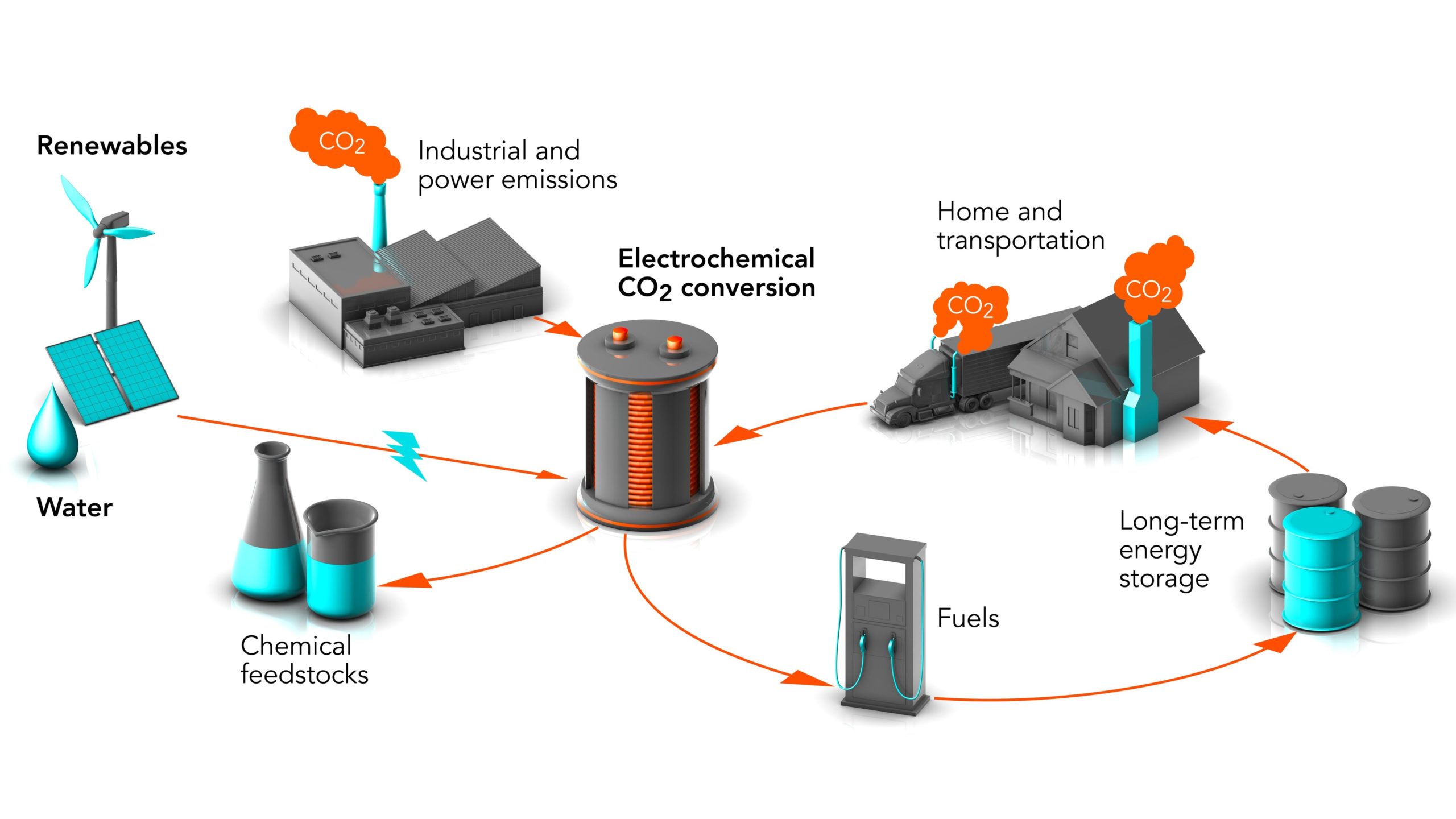
Electrochemical Reduction of CO2. The electrochemical reduction of CO2 is a promising technology that allows the conversion of carbon dioxide emissions into valuable products through the application of electricity. This process involves the use of an electrolytic cell that uses renewable electricity to reduce CO2 to form chemical products like hydrocarbons, alcohols, and other chemicals. Compared to traditional methods of CO2 reduction, the electrochemical process has several advantages, including mild reaction conditions, high selectivity for desired products, and the potential to use intermittent renewable electricity sources. However, there are several challenges that need to be addressed, such as improving the efficiency of the process, reducing its cost, and addressing issues related to scalability. One of the main challenges in the electrochemical reduction of CO2 is to develop efficient and selective catalysts that can promote the desired chemical reactions. Researchers are exploring different catalyst materials, such as metals, metal alloys, and metal oxides, to improve the performance of the electrochemical cells. Another challenge is the integration of renewable energy sources, such as solar or wind power, to power the electrochemical cells. The use of renewable energy sources can increase the sustainability of the electrochemical reduction of CO2 by reducing the carbon footprint of the process. The electrochemical reduction of CO2 has the potential to revolutionize various industries, including transportation, chemical production, and agriculture, by reducing their carbon footprint. Moreover, this technology can contribute to climate change mitigation by reducing greenhouse gas emissions. To achieve the widespread adoption of this technology, supportive policies and regulations are required to incentivize investment in research and development, and to facilitate the deployment of electrochemical reduction facilities. In conclusion, the electrochemical reduction of CO2 is a promising technology that offers several advantages over traditional methods of CO2 reduction. Research efforts in this field are advancing rapidly, and with continued innovation and investment, this technology has the potential to contribute significantly to a more sustainable and low-carbon future.
Electrochemical Conversion of Biomass

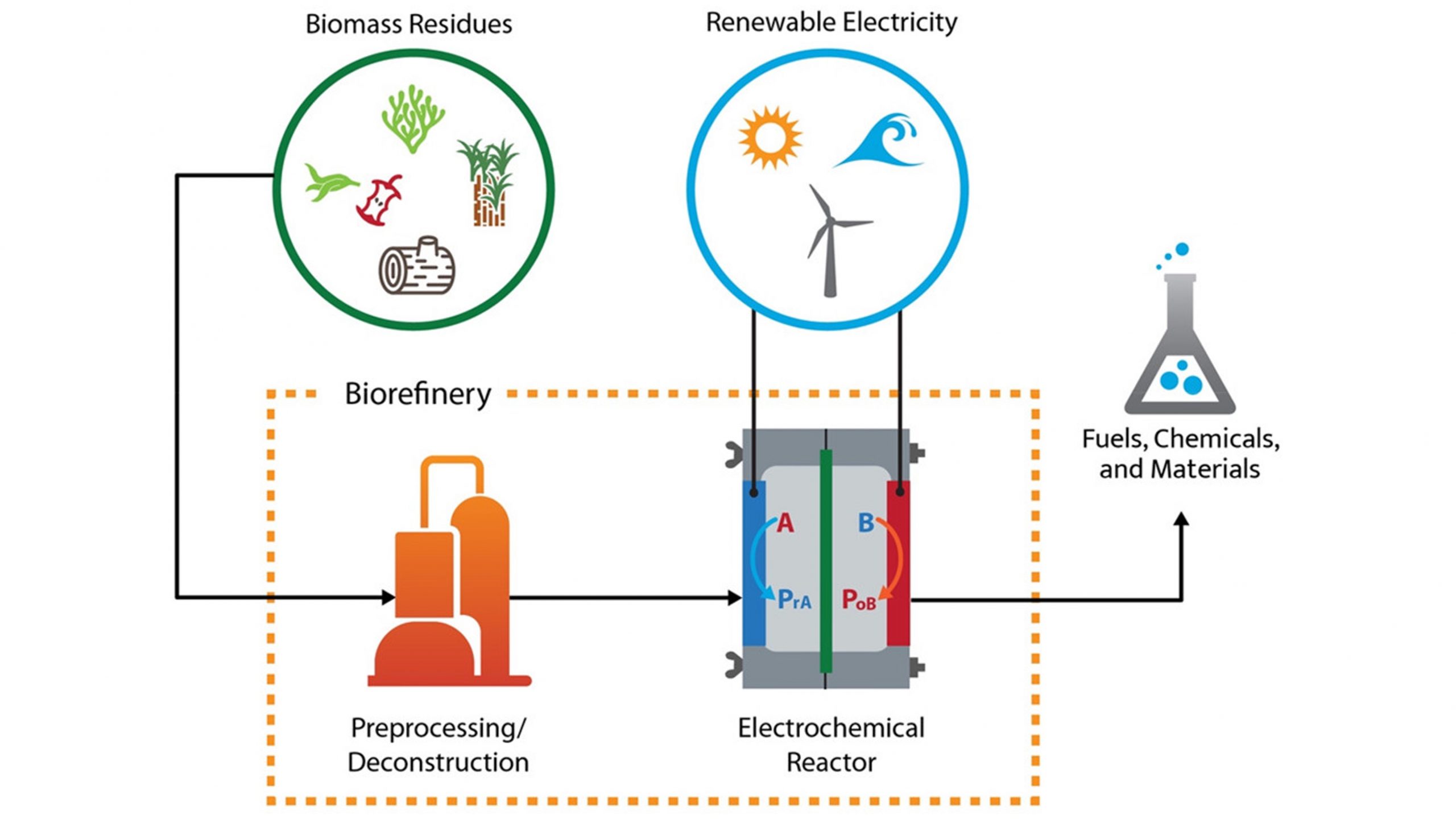
Electrochemical Conversion of Biomass. Electrochemical conversion of biomass is a promising field that holds great potential for sustainable energy production and waste management. It involves the utilization of electrochemical processes to transform biomass-derived compounds into valuable products. This approach offers several advantages, including high energy efficiency, scalability, and the ability to convert a wide range of biomass feedstocks. The process typically starts with the extraction of biopolymers, such as cellulose, hemicellulose, and lignin, from biomass sources like agricultural residues, wood waste, and dedicated energy crops. These biopolymers can be further processed into monomers and converted into biofuels, platform chemicals, or other value-added products using electrochemical methods. The electrochemical conversion can occur through various pathways, including electrolysis, electrocatalytic reactions, and microbial electrochemical systems. By employing catalysts, the conversion efficiency and selectivity can be enhanced, leading to higher yields of desired products. Furthermore, electrochemical conversion offers the advantage of operating under mild conditions, reducing the need for harsh chemicals or high temperatures that are typically associated with conventional biomass conversion methods. This aspect not only contributes to energy savings but also reduces the environmental impact of the overall process. Additionally, electrochemical conversion can play a vital role in addressing the challenge of waste management by utilizing organic waste streams and converting them into valuable resources. The process can contribute to the circular economy by closing the loop on biomass utilization and reducing reliance on fossil fuels. The versatility of electrochemical conversion allows for the production of a wide range of products, including bioethanol, hydrogen, organic acids, and other specialty chemicals, depending on the specific feedstock and reaction conditions. Electrochemical processes can also be integrated with other renewable energy technologies, such as solar and wind power, to create synergistic systems that maximize energy production and utilization. Moreover, the electrochemical conversion of biomass aligns with the goals of mitigating greenhouse gas emissions and combating climate change by reducing reliance on fossil fuels and promoting the use of renewable resources. As the field of electrochemical conversion of biomass continues to advance, research and development efforts focus on improving process efficiency, developing novel catalysts and materials, and optimizing reactor design. The integration of electrochemical conversion with emerging technologies, such as artificial intelligence and machine learning, holds great promise for further advancements and process optimization. Overall, electrochemical conversion of biomass represents a sustainable and promising pathway towards a greener future, offering a viable solution for renewable energy production, waste valorization, and the transition towards a circular bio-based economy.